There is a lot of talk this year about high yield corn programs and the need to assess plant nutrient levels in-season. Regardless of the interest in higher yield corn production systems, my premise has been that we should use tissue testing as part of an economically sustainable production system, whether we are aiming for record yields or not. I guess you would say I was a supporter of plant tissue testing before it was the “cool” thing to do. I have maintained an active tissue testing program in previous seasons, usually working with 20-30 corn growers on their farms as well as collecting replicated plot data in Nitrogen response trials. I have found the easy part of this is “how-to” collect a plant tissue sample, but the more important part is how-to implement a plant tissue testing program.
The easy part is collecting the sample and specific instruction are included in the following file. For corn over 12″ tall, collect the most recently mature leaf, or the top “collared” leaf. At this time of year I collect 15-20 leaves to make a sample. I usually collect 1 sample per 40 acres. Later in the season, when the ear is apparent, collect the ear leaf. When leaves are larger I can usually only fit 12-15 leaves in a sample bag. For sampe consistency, break off the portion at the base of the leaf that wraps around the stalk. I have heard other people that use 1 leaf above or below the ear. I don’t think there is a wrong way, but I recommend consistency in sampling.
Sampling-for-Corn-Plant-Tissue-Analysis.pdf
More important to me is the when you sample and what you do with the results. What I do is not the only way, but I hope to emphasize the consistency in sampling practices. If you will develop consistent protocols on your farm, it is easier to compare among fields and determine deficiencies relative to other fields. My goal each season is to collect a sample at 40 and 60 days after planting for every field or each 40 acres.  The sample at 40 days after planting is at “lay-by.” Remember that the ear shoot is formed at V6-V8 growth stage. This means that maximum ear size, both length and rows is being determined at this time Therefore, I think this is a very important growth stage to assess nutrient levels. Essential nutrients such as Zinc may be deficient early with no visible symptoms. By the time the root system has expanded later in the season, tissue levels will be sufficient, and late sampling would give no indication of early season deficiency.
The sample at 40 days after planting is at “lay-by.” Remember that the ear shoot is formed at V6-V8 growth stage. This means that maximum ear size, both length and rows is being determined at this time Therefore, I think this is a very important growth stage to assess nutrient levels. Essential nutrients such as Zinc may be deficient early with no visible symptoms. By the time the root system has expanded later in the season, tissue levels will be sufficient, and late sampling would give no indication of early season deficiency.
I try to collect a second sample at 60 days after planting. This is usually several days prior to tasseling. I have three specific reasons I like this timing; 1. Fertilizer applications are completed, 2. If nutrient levels are low, applications can be made before the critical period of tassel emergence and pollination, and 3. We know this is a time of peak nutrient demand.
In our sandy soils I am mostly concerned with the level of plant tissue Nitrogen. Nitrogen is the most mobile of our macronutrients, and we know corn has a large yield response to nitrogen sufficiency or deficiency. The attached graph shows a series of sampling dates and fields at an area farm. You can clearly see that nitrogen level is very temporal, that it changes significantly over time. 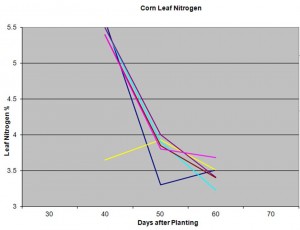 This should emphasize why I believe it is critical to develop consistency in sampling. This example shows 6 fields that were sampled at 40, 50, and 60 days after sampling. In this example, you see a relatively consistent trend among these samples. However, you also see that one field had low N levels early season at day 40, one field that had significantly lower levels than the others at day 50, and another field that had the lowest level of leaf N at day 60. Each of these samples can help us make management decisions throughout the season, while a single sample at tassel would provide very little information. However, none of these samples would have been deemed deficient. Each sample alone, without comparison to the entire series of samples would not be very valuable. Likewise, you can tell that the N level was more influenced by maturity than field. I usually make similar comparisons with Potassium, Magnesium, or other nutrients of concern.
This should emphasize why I believe it is critical to develop consistency in sampling. This example shows 6 fields that were sampled at 40, 50, and 60 days after sampling. In this example, you see a relatively consistent trend among these samples. However, you also see that one field had low N levels early season at day 40, one field that had significantly lower levels than the others at day 50, and another field that had the lowest level of leaf N at day 60. Each of these samples can help us make management decisions throughout the season, while a single sample at tassel would provide very little information. However, none of these samples would have been deemed deficient. Each sample alone, without comparison to the entire series of samples would not be very valuable. Likewise, you can tell that the N level was more influenced by maturity than field. I usually make similar comparisons with Potassium, Magnesium, or other nutrients of concern.
It has been my experience that most farms have one sampling day, and collect samples from fields that range from about 50 days after planting to 70 days after planting that day and make broad interpretions. I think it is easy to see from the patterns here that there may be more to the story than you would find by sampling the whole farm on one day. I hope you understand the importance of consistency in sampling whether you are seeking record yields, or simply to optimize production. The time is here for tissue sampling in North Florida. I have sampling kits ready to go to the plant testing lab of your choice. If I can be of help, contact me at the Extension Office.
